Sona Nanotech

Overview
Halifax, Nova Scotia-based Sona Nanotech’s (CSE:SONA,OTCQB:SNANF) patented nanoparticle manufacturing processes have allowed it to develop an innovative cancer therapy that can shrink tumors from the inside out, and in so doing, enables standard immunotherapy drugs to work significantly better and potentially on patients for whom no other treatment has worked. Sona’s gold nanorod manufacturing technology uniquely does not rely on the use of toxins, making them an ideal agent to be intratumorally injected in humans which can be used to generate therapeutic heat in the tumor.
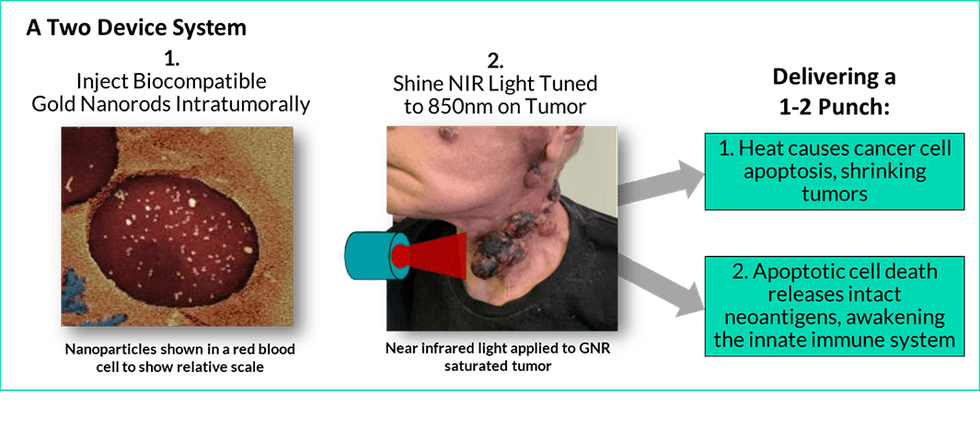
Sona’s therapy will be applied to the late-stage melanoma tumors that cannot safely be resected and for which no other therapy has worked. Once Sona’s gold nanorods are injected into a tumor, Sona’s laser directs non-thermal, near infrared light energy that is tuned to the same wavelength as the gold nanorods. The gold nanorods react by vibrating, turning the light energy into heat, killing the cancer cells selectively and causing apoptosis in their cells, resulting in the release of new antigens into the system. These new antigens prime the innate immune system permitting immune-oncology drugs to be more effective at eliminating the treated tumor and can serve as a tumor specific vaccine for the body, attacking distant tumors that were not treated, acting much like a vaccine does.
Sona’s board includes the key builder of vaccine maker Sanofi-Pasteur, a Khosla Ventures biotech PhD CEO and a long-time president of Medtronic Canada. Its management team includes an experienced capital markets CEO, CSO with a PhD, and a CMO who is a highly experienced surgical oncologist and researcher.
Background
Sona Nanotech is advancing nanotechnology-based healthcare through its proprietary, biocompatible gold nanorod manufacturing technology that has the potential to change the face of cancer therapy.
Modern nanotechnology has some very real — and very promising — applications in medicine. It can help make novel diagnostic instruments and imaging significantly more effective. It can help physicians deliver treatments and medications with greater precision and improved safety. And, when combined with other technologies such as predictive analytics and artificial intelligence, nanotechnology can offer a better prognosis, diagnosis, and treatment for a multitude of complex conditions. Sona Nanotech believes it can also be used to beat cancer.

Most of these use cases are made possible through a material known as a nanoparticle, a near-atomic-scale piece of matter that can be manipulated in a variety of different ways.
Sona Nanotech has, for the past several years, been diligently developing and operationalizing its biocompatible nanoparticle technology for use in targeted cancer therapy.
Biocompatibility is key to the application of nanotechnology in medicine due to concerns of toxicity for anything to be administered in vivo. Because of the promise of nanomedical devices and materials, both medical and regulatory agencies are embracing the technology and have already developed frameworks to assess their impact on the human body.
Sona Nanotech’s technology platform leverages its patented and uniquely biocompatible gold nanorods (GNRs). Measuring between 10 and 100 nanometres in length, these rod-shaped nanoparticles are manufactured by chemical synthesis and can be conjugated to a multitude of different molecules. They can also be conjugated to a multitude of different molecules and agents, giving them a range of potential applications, including for targeted drug delivery, diagnostic imagery, immunoassay and biosensing.
Company Highlights
- Last year’s acquisition of Siva Therapeutics has allowed Sona to advance the development of its Targeted Hyperthermia Therapy (THT), which aims to address the risks of current cancer treatments by targeting cancer cells selectively while minimizing harm to healthy tissues.
- The company recently received its final report from the U.S. National Cancer Institute’s Nanotechnology Characterization Laboratory which validated Sona’s polymer-coated gold nanorods as containing no detectable endotoxins or microbial contamination.
- THT has shown promising results in reducing tumor volumes and increasing survival in small animal studies. Sona’s initial target for this technology is a locally advanced stage, irresectable melanoma.
- In addition to THT, Sona is also focused on developing rapid diagnostic tests, including for bovine tuberculosis detection and concussion screening.
- Future applications for Sona’s technology could include targeted drug delivery, photothermal cosmetic therapy, cell imaging and additional proprietary testing solutions.
- Sona has an experienced leadership team and board of directors from the healthcare and biotechnology sectors.
- The company has a clear roadmap from its current preclinical studies towards regulatory submissions, human clinical studies, and commercialization of its treatment methodology.
Key Technology: Gold Nanorods
Unlike traditional GNRs produced with cetrimonium bromide, Sona’s patented GNRs are synthesized without toxin, enhancing their biocompatibility profile and making them ideal for advanced nanomedical applications, in vivo. Biocompatibility is key to the application of nanotechnology in medicine due to concerns of toxicity for anything to be administered in vivo. Because of the promise of nanomedical devices and materials, both medical and regulatory agencies are embracing the technology and have already developed frameworks to assess their impact on the human body.
Sona’s GNR biocompatible GNR manufacturing technology is patented and Sona has submitted a provisional patent application with the United States Patent and Trademark Office, for its proprietary photothermal light device. A prototype of Sona’s medical laser was engineered to apply non-thermal, 860-nanometer wavelength high-intensity infrared laser light. The device has been designed for use with Sona’s GNRs that efficiently convert its non-thermal light energy, which can pass harmlessly through up to 1.5 cm of healthy cells, into heat.
Targeted Hyperthermia Therapy (THT) – Overview
Current cancer treatments are risky, expensive and potentially harmful. Chemotherapy and radiotherapy, for instance, are completely non-selective in cell destruction. Advanced therapies are frequently beyond the price point of most patients, while manual surgery is risky, particularly for the infirm. Sona’s Targeted Hyperthermia Therapy (THT) represents a potential solution to all three of these problems.
THT Highlights
- Precise: THT uses moderate heat which kills cancer cells selectively, without damaging healthy cells, as it happens with radiation, ablation, chemo and surgery.
- Vetted: Sona’s platform, gold nanorod manufacturing technology has been vetted by the U.S. National Cancer Institute’s Nanotechnology Characterization Laboratory as being biocompatible, making it the ideal gold nanorod for use in humans.
- Preclinically Proven: Sona’s therapy has succeeded in shrinking tumors in multiple mouse studies and is now poised to undertake the safety studies needed to permit a first-in-human study.
- Immune System Booster: Sona’s therapy causes a breakdown of cancerous cells which present antigens not previously seen by the immune system, causing it to awaken and engage the neoantigen. This priming of the immune system has been shown to enhance the response of immunotherapy drugs, of which US$50 billion are sold each year.
- Clear Path Forward: The company has a clear roadmap from its current preclinical studies towards regulatory submissions, clinical studies and commercialization, with the goal to be in humans in the first half of 2025.
THT’s ‘hyperthermia’ approach heats tumors to 41-48°C – enough to kill cancer cells, but not enough to harm healthy cells. THT promotes apoptosis, a type of cell death in which a series of molecular steps in a cell lead to its death.
First Target Indication: Advanced, Irresectable Melanoma
There are no current cancer treatments shown to consistently work in reversing late-stage melanoma that cannot be safely resected. The preferred treatment of the National Comprehensive Cancer Network guidelines (v. 2.2023) calls for systemic therapy which raises concerns about toxicity given the volume of drugs needed to permeate the entire body, when it is only desired to be in the tumor. Sona’s THT therapy will be a localized, intratumorally administered adjuvant to immunotherapy, thereby mitigating systemic toxicity concerns and leveraging and empowering immuno-oncological drugs.
Current Preclinical Efficacy Study – Early Results
Sona recently received positive interim results from its triple negative breast cancer mouse model study with the Giacomantonio Immuno-Oncology Research Group at Dalhousie University, indicating that tumors treated with a single THT treatment shrunk within the first 24 hours, with an average reduction in size of 80 percent compared to matched controls. The results also showed that THT created a synergistic effect when combined with a current leading cancer immunotherapy agent, with the combination working much more effectively than either THT or the drug alone. The combination of the two also activated a systemic immune response that caused distant, untreated tumors to also shrink. This response is indicative of an ‘abscopal effect’ in which a local treatment stimulates a broad response, and it could lead to the development of personalized cancer vaccines.
The preclinical results suggest that Sona’s THT therapy can be a complete game changer in the fight against cancer.
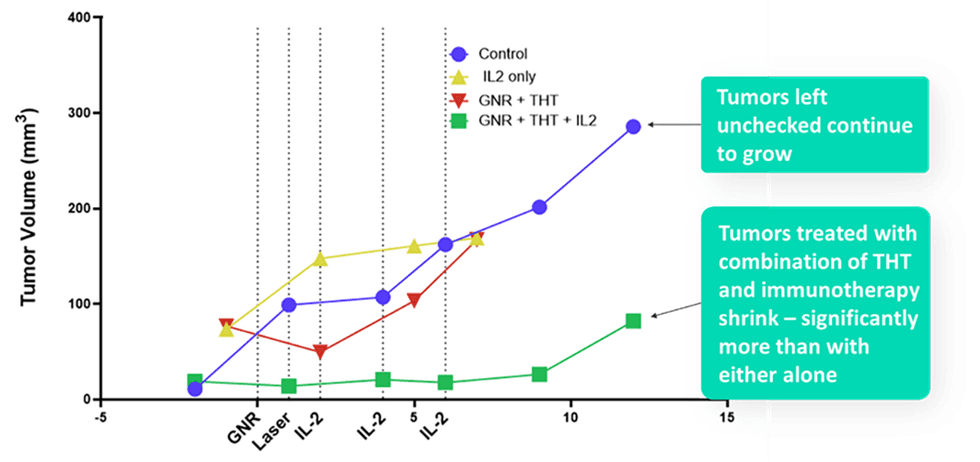
The findings also provide early evidence for increased local and distant immune reactivity following tumor GNP activation and Interleukin-2 (IL-2). Within 12 hours of receiving IL-2 plus a single administration of intratumorally-activated GNRs, there was a significant, 80 percent reduction in tumor volume, though with some regrowth overtime, which will be explored in scheduled longevity studies. There was a transient decrease in tumor volume with activated GNRs alone. The results also showed that THT created a synergistic effect when combined with IL-2, with the combination working much more effectively than either THT or the drug alone. (see green line in Figure 1 below)
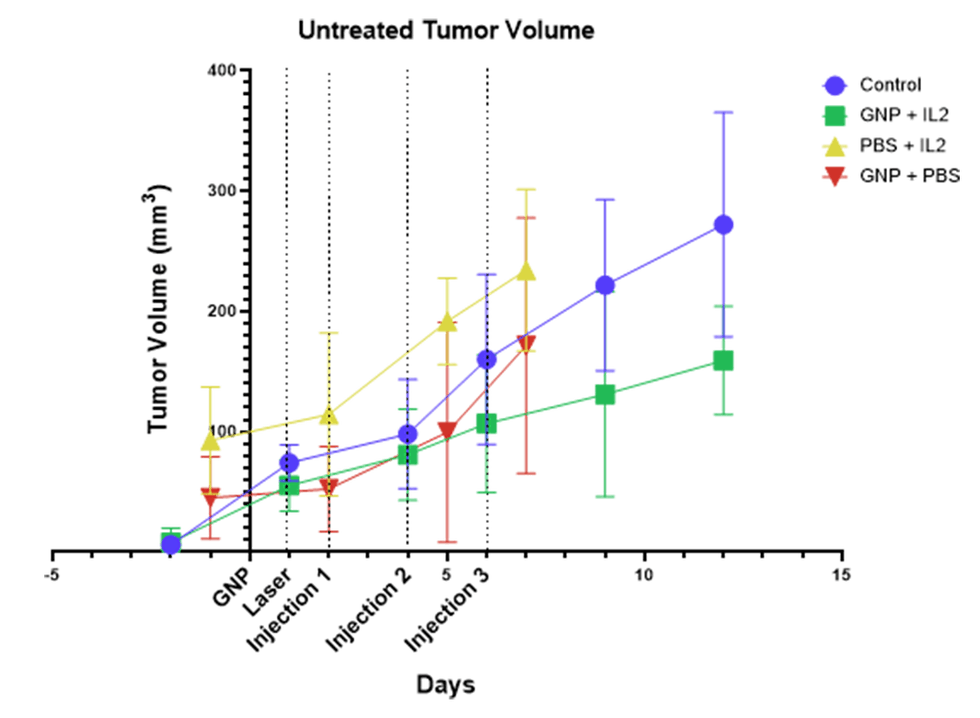
An immune response was also demonstrated in lymph nodes using a dual flank model (See
figure 2), with the left flank being the treated flank and the right, the observed flank. IL-2 plus THT showed immune stimulation in the lymph node on the treated side (LLN) with
significant increases in CD8+ T-cells and natural-killer (NK) cells compared with the right flank. This evidence shows the activation of a systemic immune response that caused distant, untreated tumors to also shrink. This response is indicative of an ‘abscopal effect’ in which a local treatment stimulates a broad response, and it could lead to the development of personalized cancer vaccines.
Sona is well-positioned to address the challenges of fully developing its THT therapy. Its GNR materials have already undergone a multi-stage independent assessment by the US National Cancer Institute’s Nanotechnology Characterization Laboratory. The results, thus far, have been excellent, with highly uniform particle size and no microbial contamination or endotoxins detected in Sona’s GNRs, making them potentially ideal for in vivo applications.
Sona has submitted a provisional patent application with the United States Patent and Trademark Office, for its proprietary photothermal light device. A prototype of Sona’s medical laser was engineered to apply non-thermal, 860-nanometer wavelength high-intensity infrared laser light. The device has been designed for use with Sona’s patented biocompatible gold nanorods which efficiently convert the non-thermal light energy into heat.
It’s not just Sona’s proprietary technology that makes it so promising, either. The company’s management team and advisory group includes experts from across the scientific and healthcare industries. One of the most notable is scientific advisor, and the principal investigator for Sona’s current THT efficacy study, Dr. Carman Giacomantonio, a surgical oncologist and researcher at Dalhousie University, who recently signed on to be Sona’s chief medical officer.
Finally, its board of directors also includes Mark Lievonen, co-chair of Canada’s COVID-19 Vaccine Taskforce and former CEO of vaccine maker Sanofi-Pasteur; Walter Strapps, CSO of Khosla Ventures’ Liberate Bio; and Neil Fraser, who was a longtime president of Medtronic Canada and a current member of Health Canada’s Advisory Panel on Health Innovation.
Management Team
David Regan – CEO
As the chief executive officer of Sona Nanotech, David Regan has led Sona since 2020 through four financings, the acquisition of Siva Therapeutics, the development of its cancer therapy, and is responsible for business and commercial operations oversight. A strategy consultant and corporate director, Regan has over 20 years of public company experience in strategy, IR and corporate development. He holds an MBA from INSEAD and a BBA with Honours from St. Francis Xavier University.
Dr. Carman Giacomantonio – Chief Medical Officer
Dr. Carman Giacomantonio is a practicing surgical oncologist, professor of surgery and principal of The Giacomantonio Immuno-Oncology Research Group. Giacomantonio’s research focuses on the impact of surgical procedures, specifically biopsies on the tumor microenvironment and subsequent development of cancer metastases, and ways to mitigate this risk through targeted therapies. His lab is also studying the mechanism of action of interleukin-2 therapy in the treatment of melanoma and other cancers.
Dr. Len Pagliaro – Chief Science Officer
Formerly a professor of bioengineering and laboratory medicine at the University of Washington, Dr. Len Pagliaro is the chief science officer of Sona Nanotech, with over two decades of experience with biotechnology products, services and licensing. During his four years at Biolmage, he worked on a commercialization strategy that took the company from concept to a $26-million P&L in just four years.
Darren Rowles – Head of Diagnostics
Darren Rowles has 17 years’ experience with nanoparticle diagnostics with a company that grew nanoparticle sales from $200,000 to $5.5 million with a ~$ 4 million profit. In addition to his role at Sona, he is an advisory board member to Gold Conference and multiple university collaboration projects. Rowles has an MBA from Bath University and a B.Sc. in biomedical science and toxicology from Cardiff Metropolitan University.
Kulbir Singh – Co-founder/Head of GNR R&D
A named author on 35 research papers and two patents, Dr. Kulbir Singh is responsible for GNR development. He holds a PhD. in chemistry from Guru Nanak Dev University. Singh is also co-founder of a science-based consumer products company.
Robert Randall – CFO
A registered CPA, Robert Randall has extensive public company experience as a CFO with organizations including Torrent Capital (CVE:TORR), Antler Gold (CVE:ANTL) and ExeBlock Technology (CSE:XBLK). He holds a bachelor’s degree in commerce from St. Mary’s University with a chartered accountant designation with Coopers and Lybrand Chartered Accountants dating back to 1987.
BOARD OF DIRECTORS
Mark Lievonen – Chairman
Formerly the president of Sanofi Pasteur, Mark Lievonen serves as co-chair of the Government of Canada’s COVID-19 Vaccine Task Force. He is also a director of OncoQuest Pharmaceuticals (KRX:078590), Biome Grow (CNSX:BIO) and the Gairdner Foundation. Lievonen holds both an MBA and an FCPA.
Walter Strapps – Director
Walter Strapps is the CSO of Liberate Bio, a biotechnology company using machine learning, advanced chemistry and molecular biology techniques to identify novel delivery vehicles for oligonucleotides. He has also served as CEO of Carver Biosciences, chief scientific officer at Gemini Therapeutics (NASDAQ:GMTX) and head of discovery at Intellia Therapeutics (NASDAQ:NTLA), in addition to working with RNA Therapeutics. Strapps holds an MA, M. Phil. and PhD.
Neil Fraser – Director
A member of the Canadian Chamber of Commerce’s Life Sciences Strategy Council, Neil Fraser is also the former president of Medtronic Canada. He is part of Health Canada’s advisory panel on health innovation, which is chaired by Dr. David Naylor. Fraser is also a director at CloudMD (CVE:DOC).
James Megann – Director
James Megann has 25 years of experience in venture capital, capital markets and marketing. As managing director of Numus Financial, he has overseen over $1.5 billion in transactions. He also serves on the board of Torrent Capital (CVE:TORR).
Source : https://investingnews.com/stocks/cse-sona/sona-nanotech/
